Research in my group is focused on the development of new or under-utilized reactions that generate molecules resembling natural products. We also use computational resources to explain the stereoselectivity of a particular reaction or to help understand why two seemingly similar molecules react in very different ways. There are two main projects on which we are currently working.
| Alkaloids (molecules containing a basic nitrogen and that are produced by plants) are an important class of molecules. Many important medicines are either isolated directly from plants or are based upon alkaloid natural products. For example, morphine is an analgesic isolated from the opium poppy. Pergolide is an anti-Parkinson's medication derived from lysergic acid.
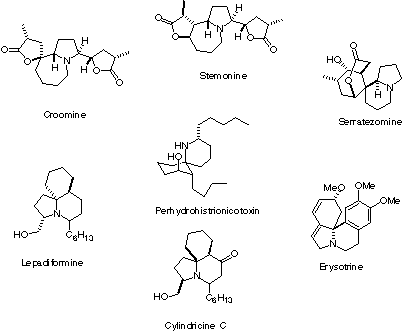
Many alkaloid structures consist of a "core" structure that is common to many compounds. Often, this core has a nitrogen-containing ring. Because many different compounds can be made from the same core structure (say, a 5-membered nitrogen-containing ring), methods for making these core structures can find a lot of use in pharmaceutical research. To the right are some representative alkaloid natural products. These and similar molecules provide the inspiration for research in my laboratory. We are specifically interested in finding new ways to quickly make highly substituted 5-membered nitrogen containing rings (pyrrolidines).
|
 |
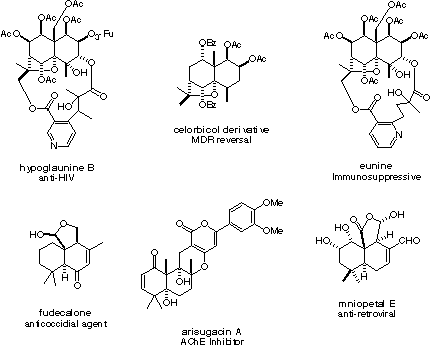 |
Terpenoids (molecules that are based upon a terpene core) are another class of natural products that have inspired drug development. Many of these compounds have a densely functionalize decaline core (two six membered rings fused together), and they often have a lot of oxygen substituents. One of the challenges in devolping ways to make these molecules is building the core structure so that substituents can easily be appended in a stereoselective way. Many of these compounds also contain quaternary carbons (carbons with 4 other non-hydrogen atoms attached) that are often difficult to construct.
To the left are some terpenoid natural products and the biological activity they posses. We are specifically interested in finding ways to quickly build the highly substituted decaline core.
|
Students are involved in every aspect of these projects. They use standard synthetic organic chemistry techniques to build, purify, and identify the molecules of interest.
Work on the pyrrolidine project has been funded by a grant from Research Corporation.
Work on the terpene project has been funded by a grant from the ACS-PRF.
|